UK’s Business Secretary Alok Shama says the government has put aside £38m towards building a “rapid deployment facility” that will ensure a coronavirus vaccine is widely available in the UK and this could start with having thirty million doses of the Oxford University vaccine candidate by September.
“This new money will help mass produce the Oxford vaccine so if trials are successful we have dosages to start vaccinating straight away,” he says.
Speaking at the daily Number 10 briefing Sunday, he said the vaccine trial in Oxford University is progressing well describing the speed at which the trials have been organised as “genuinely unprecedented”.
According to the minister, a licensing agreement with AstraZeneca has been reached allowing for the manufacture of 30 million doses to be available by September.
Coronavirus Will Disappear On Its Own, Without Vaccine – Trump
“The UK will be first to get access,” he says.
However, echoing warnings made by other authorities and experts, he says “it’s possible will never find a successful coronavirus vaccine”.
Sharma added that six drugs aimed at fighting the virus have now entered clinical trials, adding that “in order to definitively conquer this disease we need to find a safe workable vaccine”.
Businessday learnt that the keenly-watched COVID-19 vaccine being developed at Oxford, will be priced to allow as wide as possible access to it, if it proves successful, and will be made at huge scale to keep costs down and supply up.
The Oxford University professor co-leading its development, Adrian Hill, says ensuring wide distribution and low cost have been central to the project from the start.Microbiologist Elisa Granato, being injected as part of the first human trials in the UKootential coronavirus “This not going to be an expensive vaccine,” Hill told Reuters in an interview. “It’s going to be a single dose vaccine. It’s going to be made for global supply and it’s going to be made in many different locations. That was always our plan.”
Coronavirus: NAFDAC Approves Chloroquine Production For Clinical Trial
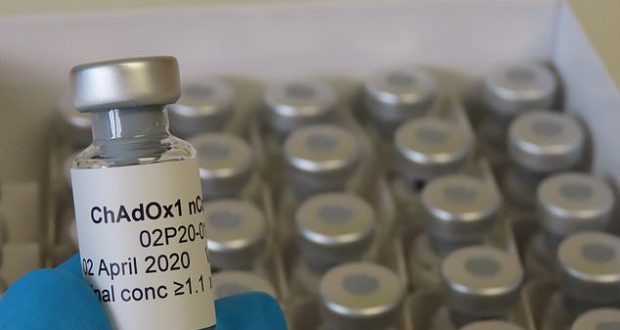
The experimental vaccine, known as ChAdOx1 nCoV-19, is one of the front runners in the global race to provide protection against the new coronavirus causing the COVID-19 pandemic.
Hill’s team began early-stage human trials of the vaccine last week administering doses to candidates in the UK, making it one of only a handful to have reached that milestone. As of this week, more than 1,000 people have been dosed in the trial – with around half getting the experimental vaccine and the other half serving as a control group.
Preliminary data from an earlier small trial of the experimental vaccine in six monkeys found that some of the animals given a single shot developed antibodies against the virus within 14 days, and all developed protective antibodies within 28 days.
When the monkeys were exposed to the new coronavirus, the vaccine appeared to prevent damage to the lungs and kept the virus from making copies of itself there, although it was still actively replicating in the nose.
Coronavirus: This Virus May Never End ― WHO Reveals
Hill said the data from the animal tests were “encouraging of course” and reinforced his team’s high degree of confidence that ongoing human trials of the shot will also show positive results. The first signals on whether and how well it works could come as early as July or August.
Asked about the progress of the human trials, Hill said he and his team “are not going to give a running commentary” but added, “You can conclude that if the trial is still running – as it certainly is – that would mean there have been no major upsets.”
Almost 4.5 million people have been reported to be infected by the novel coronavirus globally and more than 301,000 have died, according to a Reuters tally.
Health and disease experts say a vaccine that protects people from the new coronavirus could help end the pandemic, but finding one that works and manufacturing enough doses is a huge challenge.
Coronavirus: Prominent Personalities Who Have Died Of COVID-19
The ChAdOx vaccine, a type known as a recombinant viral vector vaccine, uses a weakened version of the common-cold virus spiked with proteins from the novel coronavirus to generate a response from the body’s immune system.
Other vaccines in human trials include those by Moderna Inc, Pfizer Inc and BioNTech SE and China’s CanSino Biologics Inc. Hill told Reuters the ChAdOx1 project has at least seven manufacturing sites around the world. Those include India’s Serum Institute as well as sites in Europe and China which will ensure global manufacturing of the vaccine if the trials succeed.
Hill has said that up to a million doses of the shot are already being manufactured and will be available by September, even before trials fully prove whether it works.
“The ambition is shared to get a low-priced, very, very extensively available vaccine as soon as possible,” Hill said. “And one of the reasons that we chose Astrazeneca was because they shared that ambition and they were convincing that they could provide supply and large scale.”
Post Disclaimer
The opinions, beliefs and viewpoints expressed by the author and forum participants on this website do not necessarily reflect the opinions, beliefs and viewpoints of Anaedo Online or official policies of the Anaedo Online.